SlowMo
Case study
SlowMo is a digital therapy app for paranoia, created by an interdisciplinary team at King's College London. Compared with usual care alone, research has shown that SlowMo therapy reduced self-reported paranoia at 24 weeks.
As the first evidence-based and UK Conformity Assessed (UKCA) marked digital therapeutic for paranoia, it is cutting-edge and unique.
The therapy app allows patients and therapists to perform in-person therapy sessions and use the software daily. It helps people recognise unhelpful, quick-thinking patterns by visualising their thoughts as fast-spinning, grey bubbles. Slow-spinning, coloured bubbles shrink fears and help people feel safer and live well.
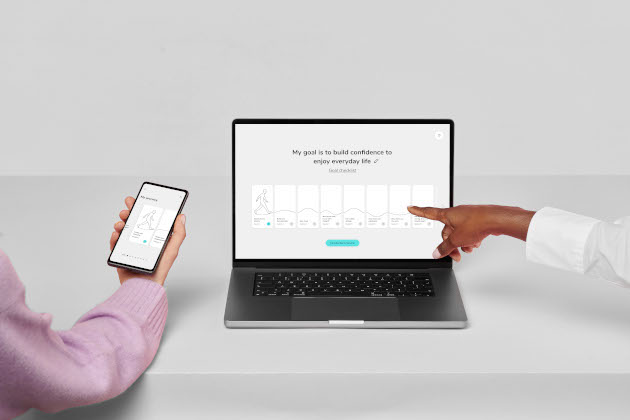
Age and ethnicity-related technological disparities did not affect participants' involvement with SlowMo, indicating that the therapy's design helped close the "digital divide".
Research clinical psychologist Amy Hardy and her team at King's College London have been developing the programme for five years, incorporating views and expertise from service users, clinicians, inclusive healthcare designers, and technologists. To develop the back-end system, Bitjam was brought on board following a controlled clinical trial.
With the £1.3 million award from the Wellcome Foundation, Bitjam, along with Special Projects, Mindtech, and CHEATA will help to evaluate the implementation and commercialisation of SlowMo's therapy, developing the app beyond its current clickable prototype form. Tests will be conducted across three NHS trusts. Within five years, it is expected to be available across the entire NHS.
Bitjam has the clinical background and technical experience to build apps and software that meet the latest MHRA requirements. Our team has a deep understanding of clinical devices and how they can be developed as well as how patients can use them. We understand the challenges you may face when developing a medical device so we make sure we work together with you from start to finish; helping you develop your product from concept to completion. Read more on our work Medical Device page.