OSI
Online Support and Intervention for Child Anxiety
Case study
OSI: Online Support and Intervention was developed by experts in treating childhood anxiety problems at the Universities of Reading and Oxford University with funding from the National Institute for Health and Care Research (NIHR).
OSI for Child Anxiety is an online, parent-led, therapist-supported psychological intervention for child anxiety. Three different versions of OSI are used across the three studies.
Co-designed by NHS clinicians and service users, OSI includes a parent website, clinician case management system, and child's game app. It is an online version of an existing evidence-based brief form of Cognitive Behavioural Therapy (CBT) that guides parents in applying CBT principles to their child's day-to-day life. In OSI, parents learn skills to help their child face their fears and deal with factors that may contribute to anxiety symptoms.
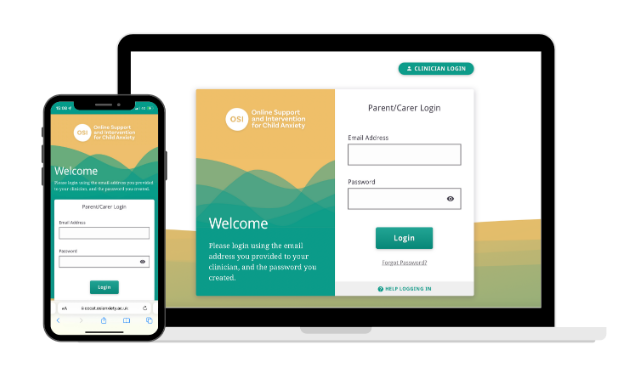
OSI consists of seven modules, released weekly, and a follow-up module. There are videos, quizzes, and interactive worksheets included in each module, as well as built-in questionnaires for parents/carers and their therapists to track a child's progress.
The clinical system houses appointment booking, homework/worksheets for parents, clinical notes, and supervision details. The game app hosted within the project encourages children to try strategies with their parents/carers.
The Bitjam team took over the hosting and development of this project from another provider and began work on improving how the digital intervention performed. This cloud-based system serves as an administrative backend for clinical staff and researcher analysis; a clinic system for client lists and client data; as well as a secure system for parent coursework and messaging.
Once the research team have completed the OSI trial period, Bitjam will support the expansion of the current version of the OSI system while simultaneously planning for the development of the next generation.
We were recommended to the Department of Psychiatry for two reasons: our track record of delivering clinical trials and the expertise we offer beyond a clinical trial's lifespan made us the ideal team to take over the project and ensure its future development.
The collaboration with a highly respected research team has been very rewarding, and we look forward to extending the functionality and lifespan of this valuable project in the future.